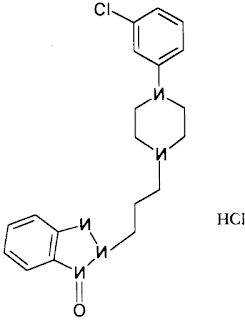
Development and validation of liquid chromatogrphic method for Trazodone hydrochloride
Abstract
A simple,
isocratic, rapid and sensitive high performance liquid chromatographic method
has been developed for quantitative determination of Trazodone Hydrochloride
and its three process related impurities. The method has been validated for the
determination of related substances in Trazodone Hydrochloride using a C18, ODS
(4.6mm×250mm×10μ m) column by keeping the flow rate of 1.5ml / min and having
sensitivity of 0.2. The elution is carried out using a mobile phase consisting
of methanol l80 ml, Acetonitrile 180 ml, Tetrahydrofuran 40 ml, and Trifluroacetic
acid (0.5%) 600 ml. The detection isbeen carried out at 252 nm with injection volume
of 10 μ L. The run time is 15 minutes for estimation of related substance. The
precision, Linearity and accuracy of the method are demonstrated for Trazodone
Hydrochloride. Specificity of the method is also been studied.



Comments
Post a Comment